RAP-A Trials
Efficacy and effectiveness of RAP-A have been supported through several randomised controlled trials that statistically analyse intervention effects and clinical significance. Below please find a description of three of those trials.
Initial efficacy trial
Shochet, I. M., Dadds, M. R., Holland, D., Whitefield, K., Harnett, P. H., & Osgarby, S. M. (2001)
Method
In a cohort-based randomised controlled trial, 134 Year 9 (14 year-old) students from a Brisbane high school participated in RAP-A. The previous Year 9 cohort (N=126) acted as a control group. Participants completed measures of depression and hopelessness at pre-intervention, post-intervention and 10-month follow-up. Group facilitators were specially trained mental health professionals or clinical psychology graduates. Recruitment and retention rates were high with 85% of students participating.
Results
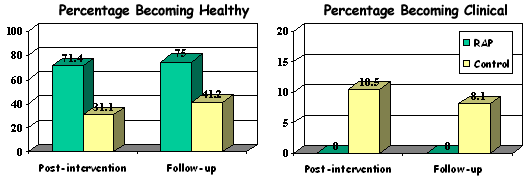
Repeated measures multivariate analysis of variance (MANOVA) showed that students in the intervention conditions reported significantly lower levels of depressive symptoms at post-intervention and 10-month follow-up, compared with the control group. Using Chi-square analyses, program effects demonstrated clinical significance, indicated by movement between healthy, subclinical and clinical ranges on depression measures. Of the adolescents who scored in the subclinical range at pre-intervention, none in the intervention groups had become clinical at follow-up, compared with 10.5% in the control condition. At follow-up, 75% of initially subclinical adolescents in the intervention condition had moved into the healthy range compared with only 41% in the control condition.
Percentages of initially at-risk adolescents moving into the healthy or clinical ranges at post-intervention and follow-up:
|
New Zealand randomised blind placebo-controlled trial
Merry, S., McDowell, H., Wild, C. J., et al (2004)
Method
A placebo-controlled trial was conducted using a version of RAP-A developed for dissemination in New Zealand (RAP-Kiwi). 392 students aged 13-15 from two schools were randomized to intervention (RAP-Kiwi) or placebo-control condition. The placebo was similar to RAP-Kiwi in time and structure, but all putative active components of the intervention (eg. CBT) were removed. Specially trained teachers conducted the groups.
Results
Analyses for this trial employed one-tailed independent-samples t-tests, ANOVA and chi-squared tests. RAP-Kiwi participants recorded significantly greater improvements in depressive symptoms at post-intervention than those in the placebo condition. In clinical terms, student movement between BDI-II minimal/mild and moderate/severe categories at post-test indicated a net improvement of eleven students in the intervention group compared with a net deterioration of three students in the placebo group. No ongoing positive effect was measured by the BDI-II, but the significant positive effect of the intervention as measured by the RADS persisted to 18-month follow-up.
Large multi-site effectiveness trial
Method
In order to test the “real world” effectiveness of RAP in a large rollout of the program using sustainable resources, a three-year multi-site effectiveness trial of RAP-A and RAP-P, funded by the National Health and Medical Research Council (NHMRC), has recently been completed. This randomised controlled trial was conducted with 2664 Year 8 students from two successive cohorts in 12 schools drawn from three Australian States. Participants were randomly allocated on a matched school basis to a control group or either RAP-A or RAP-F (in which students received RAP-A and their parents were invited take part in three RAP-P workshops and mailed a flexible delivery version of RAP-P). To control for school effects, the first cohort in each school was assigned to either control or the appropriate intervention condition (RAP-A or RAP-F) and the following cohort was assigned to the other condition. Teachers who completed a one-day RAP-A group leaders’ training workshop delivered RAP-A. RAP-P was facilitated by school counsellors, psychologists or other local mental health workers who completed the one-day RAP-P group leader training. The flexible delivery format of RAP-P consisted of six stand-alone workbooks which were mailed to parents at two-week intervals following completion of RAP-A.
Preliminary results
For the group as a whole, multi-level modelling showed an intervention effect in favour of the RAP Program at post-testing with no significance at follow-up. RAP also demonstrated clinical significance for the at-risk group. Chi-square analyses were conducted to compare the post-intervention and follow-up status of the combined RAP interventions and control group in terms of risk for depression (based on scores on the Children’s Depression Inventory). Approximately half of the at-risk students in the RAP condition were healthy at post-intervention compared with a third in the control group. This pattern of results was similar at follow-up, but statistical significance was not achieved. Looking at the effect at both time points, more than double the amount of RAP participants remain healthy at both post and follow-up (36% vs 17%).
Considered overall, the multi-site trial showed RAP to be effective at post, with a dilution at follow-up, however there are a greater proportion of at-risk students that remain healthy at both time points in the RAP group.
Sample qualitative responses from adolescents to the question “ Can you give me specific examples of how you have used RAP in your daily life?” (Posed six months post intervention by an external person)
 "When we play football, if someone starts swearing, I think don’t worry, don’t hit him yet - work things out first. Before I would have started punching way before.”
"When we play football, if someone starts swearing, I think don’t worry, don’t hit him yet - work things out first. Before I would have started punching way before.”
“With your parents and stuff, you should see their side of the story more.”
“Yes I was pretty stressed one night and I put on some really calm music and relaxed. Before I wouldn’t have done anything.
“Like not thinking negatively, like if someone stands you up, don’t think they don’t like you, just think they missed the bus or something. Don’t blame them. A friend stood me up twice...Before the program I would have been pissed and called her and just kept yelling at her. So it’s a good program because it changed my life a bit.”
“I wouldn’t use it every day, but I’ve used it before. At my best friends party there was a bit of drug use there, it got offered to me but I just passed it along and nothing was said. Before I would have probably used it if I didn’t do RAP because I probably wasn’t thinking then, like afterwards it made me think about a lot of things like that.”
![]() “I get into a lot of arguments with my brother and sister and sometimes my friends, but now I look to what will benefit me and I say, ‘okay what are they going through’ so I don’t get so upset as much. Before it always used to be my way.”
“I get into a lot of arguments with my brother and sister and sometimes my friends, but now I look to what will benefit me and I say, ‘okay what are they going through’ so I don’t get so upset as much. Before it always used to be my way.”
Summary of findings
In summary, the three trials suggest we can be confident about RAP-A’s efficacy in the short and medium term (even better than placebo). There is also evidence of effectiveness, but effects are smaller and dilute to some extent at follow up. There appears to be no additional benefits of adding the parent component to the adolescent component with regard to quantifiable impact on depressive symptoms. The RAP program appears to deliver some population health benefit in preventing depressive symptoms even when utilising sustainable local resources. Qualitative responses suggest a range of positive adjustment outcomes (such as anger management) not measured in the outcome studies to date..
 Resourceful Adolescent Program
Resourceful Adolescent Program